Una molecola chiamata astressina-B, inizialmente studiata per limitare gli effetti dello stress su stomaco e intestino, secondo uno studio della rivista Plos one, si è rivelata in grado di far ricrescere i capelli.
Gli scienziati del Centro di Ricerca per le Patologie del Sistema Digerente del’Università della California stavano infatti effettuando degli esperimenti su alcune cavie per testare l’efficacia di una nuova sostanza, battezzata astressina-B e studiata per limitare gli effetti dello stress sull'apparato gastrointestinale.
A tale scopo gli scienziati avevano creato delle cavie geneticamente modificate per produrre in grandi quantità l’ormone che induce nell’organismo una risposta agli stress.
Più alta è la sua presenza, più l’individuo si stressa facilmente.
L’astressina-B era stata pensata per intervenire nel processo di liberazione di questo ormone, bloccandone il rilascio e quindi riducendo lo stress nell’individuo.
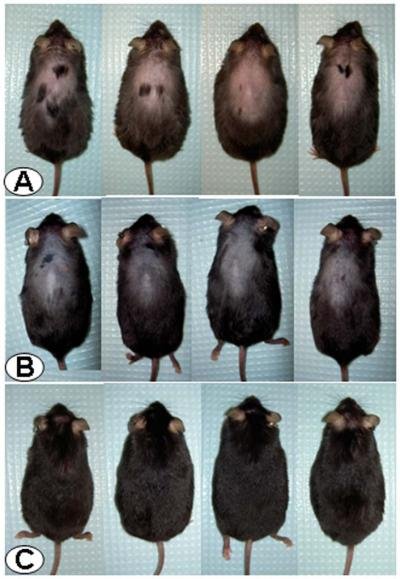
Le cavie “stressate”, che erano visibilmente malconce ed avevano iniziato a perdere il loro pelo dopo poche settimane di vita, erano state cresciute assieme ad un gruppo di controllo, dal pelo folto e scuro ed in perfette condizioni di salute.
 Giunti
al momento della sperimentazione, i ricercatori hanno iniettato per 5 giorni l’astressina-B
nelle cavie stressate e calve e li hanno quindi rimessi assieme al gruppo di
controllo.
Giunti
al momento della sperimentazione, i ricercatori hanno iniettato per 5 giorni l’astressina-B
nelle cavie stressate e calve e li hanno quindi rimessi assieme al gruppo di
controllo.
Tuttavia, una volta passati 3 mesi e giunto il momento di verificare l’impatto del trattamento sull’apparato gastrointestinale, i ricercatori non sono più stati in grado di distinguere i topi “malati” da quelli sani: alle cavie trattate con astressina-B era infatti cresciuto un folto manto peloso e ora si rivelavano irriconoscibili da quelle del gruppo di controllo.
Million Mulugeta, coordinatore della ricerca, racconta: "Abbiamo guardato dentro la gabbia e in un primo momento ci siamo chiesti perché i topi calvi non c'erano, poi li abbiamo contati, e ci siamo resi conto che a tutte le cavie era cresciuto il pelo, è stata una scoperta del tutto inaspettata".
Secondo Mulugeta questa scoperta potrebbe costituire la base fondamentale di nuovi studi sull’astressina-B mirati a farne la prima rivoluzionaria cura contro la calvizie legata a stress e invecchiamento.
E' certamente presto per parlare di rimedi anticalvizie, dato che l'esperimento è stato condotto solo su cavie, che hanno cicli di ricrescita dei peli molto diversi dall'uomo, ma i risultati finora sono incoraggianti : i topi calvi dopo cinque giorni di iniezioni di questa sostanza, hanno recuperato in poche settimane un folto manto.
L'antistressina-B è un peptide di piccole dimensioni.
”Il nostro studio ha dimostrato che un trattamento per pochi giorni ha effetti a lungo termine sulla ricrescita dei peli spiega Million Mulugeta dell’universita’ della California a Los Angeles (Ucla), nell’uomo potrebbe essere indicato nei casi di perdita di capelli dovuta a stress ed età”.
”Gli effetti sull’uomo devono essere verificati, continua il ricercatore, che nel frattempo ha brevettato l’antistressina contro la calvizie, ma abbiamo visto che gli stessi topi trattati con un farmaco usato nell’uomo per la perdita di capelli hanno avuto una ricrescita molto inferiore, il che ci fa ben sperare per le applicazioni future”.
Certamente è da aspettare gli sviluppi delle ricerche perché nulla ancora si può
dire.
Riferimenti studi scientifici
L'articolo originale della rivista PlosOne
Endocrinology, doi:10.1210/en.2007-1350
Department of Obstetrics and Gynecology (E.X., L.X.-Z., M.F.), College of Physicians and Surgeons, Columbia University, New York, New York 10032; Department of Obstetrics and Gynecology (N.R.V.), Centre Hospitalier Universitaire Vaudois, CH-1011 Lausanne, Switzerland; and The Salk Institute (J.R.), La Jolla, California 92307
Address all correspondence and requests for reprints to: Dr. Michel Ferin, Department of Obstetrics and Gynecology, College of Physicians and Surgeons, Columbia University, 630 West 168th Street, New York, New York 10032. E-mail: mf8@columbia.edu.
Administration of ghrelin, a key peptide in the regulation of energy homeostasis, has been shown to decrease LH pulse frequency while concomitantly elevating cortisol levels. Because increased endogenous CRH release in stress is associated with an inhibition of reproductive function, we have tested here whether the pulsatile LH decrease after ghrelin may reflect an activated hypothalamic-pituitary-adrenal axis and be prevented by a CRH antagonist. After a 3-h baseline LH pulse frequency monitoring, five adult ovariectomized rhesus monkeys received a 5-h saline (protocol 1) or ghrelin (100-µg bolus followed by 100 µg/h, protocol 2) infusion. In protocols 3 and 4, animals were given astressin B, a nonspecific CRH receptor antagonist (0.45 mg/kg im) 90 min before ghrelin or saline infusion. Blood samples were taken every 15 min for LH measurements, whereas cortisol and GH were measured every 45 min. Mean LH pulse frequency during the 5-h ghrelin infusion was significantly lower than in all other treatments (P < 0.05) and when compared with the baseline period (P < 0.05). Pretreatment with astressin B prevented the decrease. Ghrelin stimulated cortisol and GH secretion, whereas astressin B pretreatment prevented the cortisol, but not the GH, release. Our data indicate that CRH release mediates the inhibitory effect of ghrelin on LH pulse frequency and suggest that the inhibitory impact of an insufficient energy balance on reproductive function may in part be mediated by the hypothalamic-pituitary-adrenal axis.
|
Astressin B, a Nonselective
Corticotropin-Releasing Hormone Receptor Antagonist, Prevents
the Inhibitory Effect of Ghrelin on Luteinizing Hormone Pulse
Frequency in the Ovariectomized Rhesus Monkey
Department of Obstetrics and
Gynecology (E.X., L.X.-Z., M.F.), College of Physicians and
Surgeons, Columbia University, New York, New York 10032;
Department of Obstetrics and Gynecology (N.R.V.), Centre
Hospitalier Universitaire Vaudois, CH-1011 Lausanne, Switzerland;
and The Salk Institute (J.R.), La Jolla, California 92307
Address all correspondence and
requests for reprints to: Dr. Michel Ferin, Department of
Obstetrics and Gynecology, College of Physicians and Surgeons,
Columbia University, 630 West 168th Street, New York, New York
10032. E-mail:
mf8@columbia.edu.
Received October 1, 2007; Accepted
November 28, 2007.
Abstract
Administration of ghrelin, a key
peptide in the regulation of energy homeostasis, has been
shown to decrease LH pulse frequency while concomitantly
elevating cortisol levels. Because increased endogenous CRH
release in stress is associated with an inhibition of
reproductive function, we have tested here whether the
pulsatile LH decrease after ghrelin may reflect an activated
hypothalamic-pituitary-adrenal axis and be prevented by a
CRH antagonist. After a 3-h baseline LH pulse frequency
monitoring, five adult ovariectomized rhesus monkeys
received a 5-h saline (protocol 1) or ghrelin (100-μg bolus
followed by 100 μg/h, protocol 2) infusion. In protocols 3
and 4, animals were given astressin B, a nonspecific CRH
receptor antagonist (0.45 mg/kg im) 90 min before ghrelin or
saline infusion. Blood samples were taken every 15 min for
LH measurements, whereas cortisol and GH were measured every
45 min. Mean LH pulse frequency during the 5-h ghrelin
infusion was significantly lower than in all other
treatments (P < 0.05) and when compared with the
baseline period (P < 0.05). Pretreatment with
astressin B prevented the decrease. Ghrelin stimulated
cortisol and GH secretion, whereas astressin B pretreatment
prevented the cortisol, but not the GH, release. Our data
indicate that CRH release mediates the inhibitory effect of
ghrelin on LH pulse frequency and suggest that the
inhibitory impact of an insufficient energy balance on
reproductive function may in part be mediated by the
hypothalamic-pituitary-adrenal axis.
GHRELIN, A 28-AMINO-ACID
peptide, is the endogenous ligand of the GH secretagogue
receptor (GHS-R) and is predominantly secreted by the
stomach. Injection of ghrelin not only induces a potent GH
release but also strongly stimulates appetite in rodents and
humans. Ghrelin levels may increase during negative energy
balance, a condition also known to inhibit reproductive axis
activity. For example, high total ghrelin levels (about 1.4-
to 2.6-fold of control levels) are reported in anorexia
nervosa, a syndrome characterized by decreased food intake
and amenorrhea. Central to energy deficiency-related
reproductive dysfunction is a decreased LH pulse frequency.
This is not surprising because a proper GnRH/LH pulse
frequency is mandatory for the normal activity of the
reproductive axis. Recent data suggest that ghrelin may play
a role in mediating the inhibitory effect of a negative
energy balance on the reproductive axis. Indeed, we have
previously reported that a short-term peripheral ghrelin
infusion in the ovariectomized (OVX) monkey significantly
decreases LH pulse frequency. An acute inhibitory effect of
ghrelin on LH pulse frequency has also been observed in the
OVX rat, whereas a delay and a decrease in the amplitude of
LH pulses have been reported in the male human.
Peripheral infusion of ghrelin
has been shown to stimulate cortisol release, suggesting an
activation of the hypothalamic-pituitary-adrenal (HPA) axis
by this peptide. Intriguingly, significant increases in
cortisol levels are also observed in women showing decreases
in LH pulsatility induced by a 5-d food restriction, and
elevated ghrelin levels in patients with anorexia nervosa
are accompanied by elevated cortisol levels. These
observations suggest a potential linkage between activation
of the HPA axis and the inhibition of reproductive function
in a negative energy balance environment. Because a primary
inhibitory role of central HPA pathways in the control of
the reproductive axis during stress in the primate is well
demonstrated, we have postulated that enhanced central HPA
activity is causal to the inhibition of LH pulse frequency
that follows ghrelin infusion. To verify this hypothesis, we
have tested whether blocking endogenous CRH activity, using
astressin B (a nonspecific CRH receptor antagonist), can
prevent this inhibitory effect of ghrelin.
Materials and Methods
Animals
Five adult long-term OVX
rhesus monkeys (Macaca mulatta) (body weight
5.0–8.5 kg) were used in this study. The animals were
kept in individual cages in a temperature- and
light-controlled room (lights on 0800–2000 h) and fed
twice a day with a high-protein Purina monkey chow (Purina
Mills, St. Louis, MO) supplemented with fresh fruit or
vegetables. All animals participated in an active
enrichment program provided by the staff of Veterinary
Medicine. All procedures were approved by the
Institutional Animal Care and Use Committee of Columbia
University, and the research was conducted in accord
with the Guide for the Care and Use of Laboratory
Animals and the Animal Welfare Act.
Experimental protocols
Monkeys were briefly sedated
with ketamine (5–7 mg/kg; Ketaset, Ford Dodge, IA) in
the early morning, and catheters were inserted into both
saphenous veins for blood sampling and infusions. The
animals were then seated in a primate chair (to which
they had previously been habituated), and the experiment
was initiated about 1.5 h later, at which time they were
fully awake. Each experiment lasted 8 h and included
first a 3-h baseline control period followed by a 5-h
treatment period. Blood samples (1.2 ml) were taken at
15-min intervals for hormone measurements. During the
experiment, animals received fruits and snacks. They
were returned to their home cages after the end of each
experimental protocol and fed with their daily amount of
food.
To investigate the role of
CRH in mediating the inhibitory effect of ghrelin on LH
pulse frequency, four experimental protocols were
performed in each monkey; after a 3-h control period to
determine the baseline pulsatile LH release pattern,
animals received a 5-h saline iv infusion (1 ml/h,
protocol 1), a 5-h iv infusion of ghrelin (100 μg/h
bolus, followed by 100 μg/h, protocol 2), ghrelin
together with astressin B, a nonspecific CRH receptor
antagonist (0.45 mg/kg in one injection im 90 min before
the initiation of the ghrelin infusion, protocol 3), or
astressin B injected 90 min before a saline infusion (protocol
4). Each animal underwent all experimental protocols in
random order (except one monkey not tested under
protocol 4), and at least 2 wk elapsed between two
protocols. Human acylated ghrelin and astressin B were
synthesized in the laboratory of J.R. Doses of ghrelin
and astressin B used were shown in previous studies to
be effective in the rhesus monkey.
Assays and data analysis
Blood samples were
centrifuged, and sera were kept at −20 C until assay. LH
was measured in all samples by a recombinant homologous
RIA (reagents provided by Dr. A. F. Parlow, Pituitary
Hormones and Antisera Center, Torrance, CA) in
duplicate, as described previously. Assay sensitivity
was 0.06 ng/ml. Intra- and interassay coefficients of
variation (CV) were 4.5 and 12.6%, respectively.
Cortisol and GH were measured in every fourth sample.
Cortisol levels were assayed by RIA (Diagnostic Systems
Laboratories/Beckham, er, TX) in duplicate. Intra-
and interassay CV were 4.0 and 7.5%, respectively. All
samples from individual animals were measured in the
same assay. GH was measured by a chemiluminescent
immunoassay (Immulite System; Diagnostic Products
Corp./Siemens, Los Angeles, CA). Intraassay CV was 3.4%.
Total ghrelin levels during infusion were measured in
duplicate by RIA (Millipore Corp., St. Charles, MO) in
hourly pooled samples. All samples were measured in one
assay, with an intraassay CV of 3.3%.
Mean LH pulse frequency, LH
pulse amplitude, and LH concentrations were calculated.
LH pulse frequency during the treatment periods in the
four protocols was analyzed by one-way ANOVA with
repeated measures followed by the Newman-Keuls multiple
comparison test. The differences between baseline and
the treatment periods were compared by Student’s t
test. Three criteria were used for LH pulsatility
analysis, as described previously : 1) the LH peak
occurs within 30 min from the previous nadir; 2) the
peak level from the previous nadir must be 3-fold
greater than the intraassay CV; and 3) the LH increase
must be followed by declining levels in accord with LH
half-life. This approach to identify LH pulses was
previously verified by the Cluster pulse-detection
algorithm program. LH peaks that occurred at time 0 were
included as pulses in the baseline period, because the
treatments were initiated after the time 0 sample. Mean
cortisol and GH levels, their percent changes from
baseline at each time point, and areas under the curves
in response to treatment were calculated. Differences
between baseline and treatment and between treatments in
the four protocols were analyzed by one-way ANOVA (Kruskal-Wallis
nonparametric test) followed by the Dunn’s test. Mean
hourly ghrelin changes during ghrelin infusion were
calculated and compared by ANOVA. All statistical
analyses were performed using PRISM (GraphPad, San
Diego, CA).
Results
Mean LH pulse frequency during
the 5-h ghrelin treatment was significantly lower than that
in the other three treatments: 0.60 ± 0/h (P < 0.05
vs. 0.96 ± 0.07/h in saline and 0.92 ± 0.05/h and
0.87 ± 0.07/h in ghrelin and saline with pretreatment of
astressin B, respectively)). When compared with the 3-h
baseline period, ghrelin infusion significantly decreased LH
pulse frequency (P < 0.05); this decrease was
prevented by astressin B administration. LH pulse frequency
remained unchanged during saline infusion (with or without
astressin B pretreatment). There were no significant changes
in LH pulse amplitude or LH concentrations in any of the
treatments.
Cortisol levels significantly
increased 45 and 90 min after initiation of the ghrelin
treatment (126.0 ± 10.8% and 125.7 ± 8.1% of baseline,
respectively, P < 0.05). Pretreatment with
astressin B prevented ghrelin stimulation of cortisol
release. Mean area under the cortisol curve after astressin
pretreatment was significantly lower than that after ghrelin
alone and similar to that after saline or saline plus
astressin B.
GH levels significantly
increased 45 min after ghrelin injection (2126 ± 628.2% of
baseline, P < 0.05; n = 4). In contrast to cortisol,
astressin B did not prevent the stimulatory effect of
ghrelin on GH release. Mean area under the GH curves in
response to ghrelin infusions (with or without astressin B)
were significantly higher than saline infusion. Astressin B
itself did not show any effect on GH release .
Total ghrelin levels remained
stable during the 3-h baseline period (754.2 ± 38.8 pg/ml).
Ghrelin infusion significantly increased ghrelin levels: by
5 h, mean ghrelin had increased to 3.29-fold of baseline,
whereas overall increase over the 5-h infusion period was
1.76-fold of baseline .
Discussion
The data confirm our previous
observations that a short-term ghrelin infusion, elevating
total ghrelin levels to 1.76-fold of baseline over a 5-h
period, significantly decreases LH pulse frequency in the
OVX nonhuman primate. We also confirm that ghrelin activates
the HPA axis as shown by the increase in cortisol. Most
importantly, we report for the first time that pretreatment
with astressin B, a nonspecific CRH antagonist, prevents
this inhibitory effect of ghrelin on LH pulse frequency and
concomitantly suppresses the cortisol increase.
The pivotal observation in this
study is that blockage of endogenous CRH activity by a CRH
receptor antagonist entirely prevents the suppressive
effects of a short-term elevation of ghrelin levels on LH
pulse frequency. Because the cortisol response to ghrelin is
also prevented, it is likely that there exists a causal
relationship between an activation of the HPA axis and the
decrease in LH pulsatile activity. Because our antagonist is
specific to CRH and because other investigators have shown
that ghrelin releases CRH from rat hypothalamic explants ,
the data suggest a central role for CRH in mediating
inhibitory effects of ghrelin on pulsatile LH release. A
large body of evidence has already demonstrated a primary
inhibitory role of CRH on the GnRH pulse generator and on
the reproductive axis in several species. In the monkey,
antagonism of endogenous CRH activity also readily prevents
the decrease in pulsatile LH release after an acute stress
challenge in OVX animals and accelerates the return to
normal cyclic activity after a more chronic stress . The
results from the present study suggest, for the first time,
a mediatory role of CRH in the disruption of an essential
element in reproductive function, i.e. the GnRH
pulse generator, under conditions other than under stress
challenges, such as in a negative energy balance condition.
The overall increase in total
ghrelin levels during our ghrelin infusions was 1.76-fold of
baseline, within the reported range of total ghrelin levels
in patients with anorexia nervosa , a syndrome that includes
malnutrition as well as elevated cortisol levels. These
patients also show a decreased activity of the GnRH pulse
generator. An 85% increase in ghrelin level was reported in
amenorrheic athletes and uniquely discriminated these
subjects from those with less severe cyclic disturbances of
exercising women. In the present study in the nonhuman
primate, LH pulse frequency decreased from one pulse/63 min
during the control saline infusion to one pulse/100 min
during the 5-h ghrelin infusion, whereas CRH antagonist
pretreatment restored it to one pulse/65 min. This effect
might at first view appear modest. However, experiments in
the monkey have clearly shown that small changes in GnRH
pulse frequency in the range of those induced here by the
5-h ghrelin infusion do over a more chronic timeline
significantly interfere with the normal reproductive cycle .
In this regard, we believe that restoration to a normal LH
pulse frequency by astressin B is a physiologically relevant
observation.
Like the modest suppression of
LH pulse frequency induced by ghrelin infusions in the
present study, cortisol increases were moderate compared
with much steeper elevations observed in previous
experiments in the monkey that included acute inflammatory
stress challenges in which mean cortisol levels increased by
60% from baseline. However, this more potent activation of
the HPA axis was accompanied by a remarkably deeper
suppression of LH pulsatility. Significant in this aspect is
the demonstration by Loucks and Thuma that LH pulse
frequency inhibition after graded energy deficits is
proportional to the increase in cortisol levels. The smaller
inhibitory effect of ghrelin on LH pulse frequency in the
present experiment most probably reflects the lower degree
of activation of the HPA axis and of central CRH release and
is presumably more representative of the finer tuning
process exerted by the HPA axis on the reproductive system.
The pathways by which
peripherally secreted ghrelin stimulates the HPA axis and
whether ghrelin directly activates centrally located CRH
neurons remain to be fully investigated in both the acute
and chronic condition. However, there is good evidence
suggesting that hypothalamic neuropeptide Y (NPY)/agouti-related
peptide (AGRP) neurons mediate the effects of ghrelin. We
know, for instance, that NPY/AGRP neurons express GHS-R ,
that ghrelin administration in the rodent increases the
synthesis of both NPY and AGRP mRNA levels, and that
synthesis of these two peptides is increased in a negative
energy balance environment . Like ghrelin, both AGRP and NPY
exert a powerful orexigenic effect in the rodent and the
monkey, and the orexigenic action of ghrelin is abolished in
double-knockout NPY and AGRP mice. Data in the OVX monkey
have also shown that NPY or AGRP infusions into the third
ventricle readily inhibit pulsatile LH . These two
neuropeptides have also been shown to stimulate the HPA axis.
Finally, in the rat, NPY/AGRP neurons located in the arcuate
nucleus of the hypothalamus densely innervate
paraventricular CRH-containing neurons. Overall, the data
suggest that activation of the HPA axis by elevated ghrelin
is mediated by a NPY/AGRP-induced CRH release, which is in
turn responsible for the decline in LH pulse frequency.
Ghrelin, an acylated peptide,
was first discovered as the endogenous ligand of the GHS-R,
and as expected, our data show that there is a brief but
significant increase in GH release during ghrelin infusion,
as shown by others. In contrast to its effect on the
cortisol increase that it abolishes, the CRH antagonist has
no effect on the GH response to ghrelin, indicating that
astressin B does not interfere with the bioactivity of
ghrelin. Recently, a study has shown that chronic
administration of unacylated ghrelin, initially thought of
as an inert form of the hormone because it failed to modify
GH secretion, can fully mimic the inhibitory effect of
acylated ghrelin on LH and FSH release in the rat. These
data highlight the fact that the effects of ghrelin on the
somatotropic and gonadotropic axes are mediated by different
pathways.
In conclusion, we have
demonstrated that pretreatment with astressin B, a
nonspecific CRH antagonist, prevents the inhibitory effect
of ghrelin on LH pulse frequency. These data demonstrate
that the deleterious impact of ghrelin and possibly of a
negative energy balance on the GnRH pulse generator and the
reproductive axis is at least partially mediated by the
central HPA axis. It should be pointed out, however, that
the present data were obtained in an acute study and that a
potential role of ghrelin and of the HPA axis in long-term
situations remains to be explored. The data also suggest
that the use of a CRH antagonist may provide a novel
treatment approach in patients with reproductive dysfunction
caused by an activated HPA axis. What has not been fully
appreciated until recently in the human is the frequent
association of elevated cortisol and reproductive
dysfunction. Increased circulating and central cortisol
levels are reported in most women with the functional
chronic hypothalamic anovulation syndrome, a main cause of
infertility classically associated with lifestyle changes
that include not only energy deficiency but also psychogenic
stress and strenuous exercise. Not only is an activated HPA
axis common to functional chronic hypothalamic anovulation
syndrome patients, but metabolic aberrations have also been
frequently observed. In these patients, the role of the HPA
axis in integrating a metabolic signal, such as that from
ghrelin, in the fine-tuning process of the reproductive
system remains to be fully investigated.
This work was supported by
National Institutes of Health Grants RO1-HD-46715 (M.F.)
and PO1-DK-26741 (J.R.). J.R. is the Dr. Frederik
Paulsen Chair in Neurosciences Professor.
Disclosure Statement: N.R.V.,
E.X., L.X.-Z., and M.F. have nothing to disclose. J.R.
is founder of Sentis Medical Sciences.
First Published
Online December 6, 2007
See editorial p.
867.
Abbreviations: CV,
Coefficients of variation; GHS-R, GH secretagogue
receptor; HPA, hypothalamic-pituitary-adrenal; OVX,
ovariectomized.
|
Riferimenti bibliografici
|
![]()