Revolutionary Fast Acting Acne Medication and Treatment
Control and Treat the Root Causes
Clear Existing Skin & Prevent and Treat Acne Breakouts
Restores Your Skin's Natural Beauty

PRICES : Clearogen Complete Acne Treatment Set 2 months US$75; 2 months US$140. Clearogen Acne Lotion 1 bottle of 55ml US$32; 2 bottles of 55ml US$ 59. Clearogen Clarifyng Toner 1 bottle ok 120ml US$28; 2 bottles da 120ml US$ 52. Clearogen Foaming Cleanser 1 bottle of 125ml US$ 28; 2 bottles of 125ml US$52
Clearogen is a revolutionary new treatment for acne. Formulated by
Board Certified Dermatologist Alex Khadavi MD, Clearogen will help control and
treat acne by combining scientifically proven
natural ingredients with an FDA approved, easy 3 step treatment to attack and
both the root causes of acne as well as its symptoms.
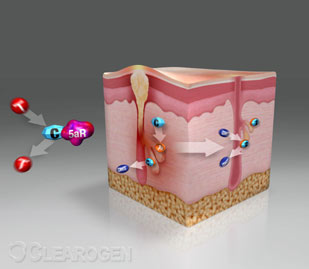
Clearogen attacks DHT (Dihydro Testosterone) the hormonal byproduct that
initiates the formation of Acne. DHT stimulates the oil glands in the skin to
produce excessive oil resulting in obstruction, infection, and inflammation of
the skin pore that are the prime symptoms of this skin condition. Clearogen
medication is a simple 3-step treatment for acne control for adults and
teenagers, used once daily for striking results.
CLEAROGEN Acne Treatment is the first
product to combine both prescription grade ingredients and scientifically proven
natural botanicals to clear acne at the first stage of formation. This easy 3
step treatment for acne attacks the root cause of acne while rejuvenating the
skin.
Clearogen is developed by Board Certified Dermatologists Dr. Alex Khadavi after
years of research and development. As a result, Clearogen uses only the safest
and most effective ingredients available and is meticulously tested to ensure
optimum results.
Clearogen is the only over-the-counter topical product to treat acnethat works
at the root of the problem. It not only treats existing acne and prevents new
lesions, but it stops the first phase of development. This is accomplished by
preventing excessive oil production by the oil glands to prevent clogging of the
pore and bacteria growth.
How Clearogen Works:
1. Clearogen Inhibits the conversion of Testosterone to DHT and reduces
excessive oil production.
2. Clearogen blocks the Androgen Receptors and prevents stimulation of oil
glands.
3. Clearogen opens the pores, kills the bacteria and reduces the inflammation,
allowing healing and skin renewal for clear and vibrant skin.
Foaming Cleanser

 Clearogen
Clarifying Skin Acne Toner eliminates pore clogging purities allowing balanced
oil production, while decreasing pore size, reducing redness, and irritation.
Botanical extracts increase skin renewal for silkier, smoother skin.
Clearogen
Clarifying Skin Acne Toner eliminates pore clogging purities allowing balanced
oil production, while decreasing pore size, reducing redness, and irritation.
Botanical extracts increase skin renewal for silkier, smoother skin.
Acne Lotion

Clearogen Acne Lotion is light weight,
shine free moisturizer that protects the skin by reducing redness,
irritation while clarifying the pore. Clearogen lotion clears existing acne
and helps prevent future breakouts before they surface on the skin, leaving
skin softer and smoother.
Other acne lotions available today are designed primarily to clear the
pimples with harsh lotions and creams that can cause redness or irritation.
Clearogen Acne Lotion clears existing skin while preventing future breakouts
by addressing the root cause of the skin condition.
Directions:
Use once daily. After cleansing and toning, apply acne lotion over the
entire face.
Ingredients:
Benzoyl Peroxide 2.5% . Deionized Water · Gamma Linolenic Acid,
Alpha Linolenic Acid . Oleic Acid . Saw Palmetto Extract . Beta Sitosterol ·
Green Tea Catechin · Caprylic/capric Triglyceride · Cetearyl Alcohol .
Dicetyl Phosphate · Ceteth-10 Phosphate · Dimethicone · Glycerin · Glycine
Soja (Soy) Sterols · Aloe Vera Gel · Glyceryl Stearate . PEG-100 Stearate ·
Sodium Hyaluronate · Cetyl Alcohol · Stearic Acid · Phenoxyethanol · Mallow
Peppermint Leaf, Primula, Veris extract · Imperata cylindrica Extract ·
Phospholipid · Comfrey Extract · Indian Plantin · Wheat Hydrolysate Extract
· Triethanolamine · Tocopheryl Acetate (vitamin E) · Neem Extract ·
Fragrance · Chamomile Extract · Xanthan Gum · Methylparaben · Propylparaben
· Sulfur · Magnesium Aluminum Silicate · Disodium EDTA · Retinoic Acid.
Acne is the most common skin condition affecting everyone at some point during their life time. Affecting both sexes and all ages from the young to the elderly. acne begins weeks before blemishes become visible on the surface of the skin, and starts within the oil producing glands. Normally these glands produce adequate amounts of oils that keep our skin healthy, shiny and young. In the presence of a body hormone known as DHT (Dihydro Testosterone), however, these glands produce excessive amounts of oil resulting in the formation of acne.
How Acne Develops:
Acne begins with the negative effects of DHT (Dihydro Testosterone) on the
oil glands.
1. The Enzyme 5 Alpha Reductase converts Testosterone to DHT .
2. DHT binds to Androgen Receptors on the oil glands.
3. DHT stimulation results in excessive oil production.
4. Excess oil obstructs the skin pores allowing bacterial growth that causes
inflammation, infection and visible acne.
Acne is a skin condition that shows up in different ways. Blemishes can include white-heads, blackheads, red bumps (pimples), and bumps that are filled with pus or cysts. What causes these annoying conditions? Skin is covered with tiny holes called pores. Pores contain sebaceous glands (also called oil glands) that make sebum (see-bum), an oil that moistens hair and skin.
Excessive oil production forms a plug called a comedone or whitehead at the opening of the pore. It fits like a cork in a bottle. This plug holds the oil and bacteria inside the oil gland which begins to swell as the skin produces more oil and bacteria. White blood cells swarm around the oil gland to kill the bacteria. The result is a pimple or pustule.
If a pore gets clogged, closes and bulges out from the skin, it is called a whitehead. If a pore clogs up but stays open, the top surface can get dark and produces a blackhead. This is not dirt and won't wash away. The color is due to an oxidation of the plug contents. Sometimes the walls of the pore are actually broken down, allowing sebum, bacteria, and dead skin cells to multiply resulting in a small, red infection called a pimple.
Most people have non-inflammatory acne, a relatively mild form with just a few whiteheads and blackheads. Non-inflammatory acne can be treated very effectively with Clearogen. For those experiencing inflammatory acne, whiteheads become infected with bacteria and swell, producing pimples and pustules. Inflammatory acne can also be treated with Clearogen.
Severe inflammatory acne, however, can cause disfiguring cysts and deep scars and needs to be under a dermatologist's care who may initially use prescription drugs like oral antibiotics or Accutane as well as laser treatments. Once the deep cystic lesions have been treated Clearogen Acne Products can be used to prevent future breakouts thereafter.
The chance of developing acne is affected by many factors. Most importantly, the rate the skin produces oil as determined by the DHT levels, plays a critical role. Because genetics strongly influence the development and persistence of acne, one's family history is important. If ones parents had severe acne, one's acne is more likely to be severe too.
Acne can change one's social life, confidence level, school and job opportunities and even lead to personality disorders. These real life situations, which are traumatic for teenagers and adults to who live with this condition. Acne affects people of all ages and teenagers seem to have the biggest challenges due to hormone changes. Parents can help teenagers by educating themselves about acne and the best methods for treating acne. Skin problems are truly more than skin deep.
About 90% of all teenagers develop acne at or about the time of puberty when hormonal levels change. During puberty that can start from the age of 12 the hormone levels began to change in the body.
Teenage
acne is directly tied to Testosterone - one of the key hormones that is
involved in adolescent puberty in both sexes. The imbalance of Testosterone
levels in adolescence results in high levels of DHT production that in turn
leads to excessive oil production within the skin and leads to teenage acne.
This is the first and the key step in formation of Acne in teenagers which
is not addressed by other treatments in the market. Instead they focus on
treating the symptoms of skin acne that are the visible lesions.
Clearogen is the first product that addresses the root cause of both adult
and teenage acne by reducing DHT production - preventing future breakouts as
well as the acne lesions.
To avoid the risk of permanent scars, it's important to treat teenage acne
as soon as the first signs appear. Clearogen is safe and effective for
everyone, and it can help prevent skin blemishes from becoming a larger
problem.
For an adult, conditions may also start as late as the 20's or 30's, especially in women whose hormones are constantly fluctuating. This is referred to as adult acne, affecting nearly half of all adult women whom develop mild to moderate conditions and ten percent of adult males.
Adult acne in women is more prominent due to the unbalanced hormone production during the menstrual cycle. In adults acne lesions typically develop before, after or during the menstruation, resulting in nagging, painful bumps under the skin, usually along the jaw-line, chin or cheeks. Clearogen Acne Product works to control the hormone production within the skin preventing the unwanted high levels of the hormone DHT that results in these lesions.
For male adult acne, Testosterone is the dominant hormone which is then converted to DHT resulting in prominent lesions throughout the skin. Clearogen acne treatment and medication controls levels of DHT in the skin clearing the existing skin and preventing acne lesions at the first stage.
|