OSH101’s hair growth effect was also compared to the hair growth effect of Rogaine®. The two groups were treated with OSH101 and Rogaine® respectively once per day for five days. After an additional sixteen days elapsed, hair growth was measured. OSH101 resulted in much faster and thicker hair growth than Rogaine®.
Another area of need is chemotherapy-induced hair loss. Many cancer patients who have undergone chemotherapy suffer from acute alopecia. It takes time for the hair to grow back, even after the chemotherapy has ended. The efficacy of OSH101 was determined in a model of chemotherapy-induced baldness. This model is well-described in the medical literature. OSH101 was applied to groups previously treated with cyclophosphamide (a widely-used chemotherapy) to induce hair loss. OSH101 stimulated extremely rapid hair re-growth compared to vehicle alone.
OsteoScreen completed a Phase I Clinical Trial for OSH101 in early 2004. The results were very encouraging. In January 2005, OsteoScreen outlicensed its hair-growth technology to Neosil, Inc., a Northern California based privately held specialty dermatology company that plans to develop the compound into an ethical pharmaceutical. (Click here to read the press release)
Details:
Indication: Male and female pattern baldness
Technology: OSH101 (drug for hair growth)
Intellectual Property: OsteoScreen holds patents for the use of OSH101 for stimulating hair growth.
Status:
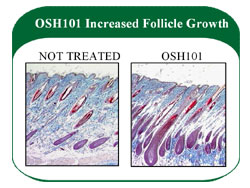
- OSH101 significantly enhanced hair growth in laboratory models by stimulating dormant hair follicles.
- OSH101 produced superior results to Rogaine® in the same models.
- OSH101 enhanced hair re-growth in a chemotherapy-induced alopecia model.
- Non-GLP pre-clinical toxicity studies showed OSH101 is safe.
- Phase I clinical trials complete.
Possible Indications:
- Male and Female Pattern Baldness
- Chemotherapy-induced alopecia.
- Alopecia areata (autoimmune skin disease resulting in hair loss).
- Age-related hair thinning.
- Post-transplant hair re-growth.