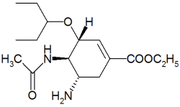
Adverse effects
Common adverse drug reactions
associated with oseltamivir therapy include: nausea,
vomiting, diarrhea, abdominal pain, and headache. Rare ADRs
include: hepatitis and elevated liver enzymes, rash,
allergic reactions including anaphylaxis, and
Stevens-Johnson syndrome.
Various other ADRs have been
reported in postmarketing surveillance including: toxic
epidermal necrolysis, cardiac arrhythmia, seizure, confusion,
aggravation of diabetes, and haemorrhagic colitis.
In November 2006, the United
States Food and Drug Administration (FDA) amending the
warning label to include the possible side effects of
delirium, hallucinations, or other related behavior. This
went further than the FDA's previous pronouncement, from a
year before, that there was insufficient evidence to claim a
causal link between oseltamivir use and the deaths of 12
Japanese children (only two were from neurological problems,
although more have died since then). The change to a more
cautionary stance was attributed to 103 new reports that the
FDA received of delirium, hallucinations and other unusual
psychiatric behavior, mostly involving Japanese patients,
received between August 29, 2005 and July 6, 2006. This was
an increase from the 126 similar cases logged between the
drug's approval in 1999 and August 2005.
In March 2007, the European
Medicines Agency said that the benefits of oseltamivir
outweighed the costs, but that it would closely monitor
reports from Japan.
In April 2007, South Korea
issued a safety warning against prescribing Tamiflu ® (marchio registrato) to
teenagers except in special cases.
For further information it
is necessary to talk to your physician.