Long-term (1-year) experience with LDS 100 in the treatment of men and women with androgenetic alopecia
Background. LDS 100 is
indicated for the treatment of men
and women with androgenetic alopecia
(male pattern hair loss, MPHL and
female pattern hair loss, FPHL).
However, the long-term (> 1 year)
efficacy of LDS 100 in this
population has not been previously
reported.
Objectives.
To assess the efficacy and safety of
LDS 100 in men and women with
androgenetic alopecia compared to
treatment with placebo device over 1
year.
Methods.
In 6 months, 240 men with MPHL and
80 women with FPHL were randomized
to receive LDS 100 treatment or
placebo treatment. Men and women
continued in up to 1 year, placebo
controlled extension studies.
Efficacy was evaluated by hair
counts, patient and investigator
assessments, and panel review of
clinical photographs.
Results.
Treatment with LDS 100 led to
durable improvements in scalp hair
over 1 year (p < 0.001 versus
placebo, all endpoints), while
treatment with placebo led to
progressive hair loss. LDS 100 was
generally well tolerated and no new
safety concerns were identified
during long-term use.
Conclusions.
In men with MPHL and in women with
FPHL, long-term treatment with LDS
100 over 1 year was well tolerated,
led to durable improvements in scalp
hair growth, and slowed the further
progression of hair loss that
occurred without treatment.
Androgenetic alopecia (male pattern hair loss, MPHL and female pattern hair loss, FPHL) occurs in men and women with an inherited sensitivity to the effects of androgens on scalp hair. The disorder is characterized by loss of visible hair over areas of the scalp due to progressive miniaturization of hair follicles. MPHL does not occur in men whit genetic deficiency of the type 2 5α-reductase (5αR) enzyme, which converts testosterone (T) to dihydrotestosterone (DHT), implicating DHT in the pathogenesis of this condition. Of the two 5αR isoenzymes in man, Type 1 predominates in sebaceous glands of the skin, including scalp, while Type 2 is present in hair follicles, as well as the prostate. In the androgenetic alopecia also occurs a reduction of the synthesis of the mRNA and the DNA with diminution of the cellular metabolism.
Three streets
of control of the hair growth exist:
- steroid control (T, 5αR, DHT);
- metabolic control (blood
circulation, glucose, ATP);
- autocrine-paracrine control (HrGF
- Hair Grow Factor).
The infrared radiation of LDS 100 (940 nm) penetrates in depth. It transits without producing great photo-biological effects; if not there where it comes to be absorbed then in the interface between the epidermis and the dermis. The photo-biological bases of therapeutic use of infrared radiation re-engage themselves in a mechanism “fallen” on various structures. There is a photoreception at the mitochondrial level. The radiation is absorbed at the level of the respiratory chain (cytochromes, oxidase cytocrome, dehydrogenase flowin) with the consequent activation of the respiratory chain and further the activation of the NAD (nicotinamide adenine dinucleotide).
At the
cellular membrane level there is an
increase in the activity of the
enzyme Na/K ATPasis, which in turn
acts on the flow of Ca+. At this
point one has a transduction and an
amplification of the stimulus in the
cellular ambit, with the activation
of the cyclical nucleotides which
modulate the synthesis of the mRNA
and the DNA.
The final photo-response is the
bio-stimulation at the various
levels of the cellular metabolic
structure. The biological activation
spreads from cell to cell with
chemical transmissions. The infrared
light increases the cellular
metabolism accompanied by an
augmentation of the capillary
vascular bed of the radiant zone
with an increase also in the supply
of oxygen.
Studies in man with MPHL and women
with FPHL showed that LDS 100 had
utility in this disorder.
Randomized placebo-controlled trials demonstrated that treatment whit LDS 100 produced significant improvements in scalp hair growth, slowed the further progression of hair loss that occurred without treatment and led to increased patient satisfaction with the appearance of their scalp hair.
LDS 100 use is contraindicated in women when they are or may potentially be pregnant and in subjects with pace-maker or other metallic devices, or those with acute phlebitis, serious arterial hypertension, neurogical illnesses, heightened cardiopathy, dermatitis and dermatosis.
Materials and methods
Study population
Men aged 18 to
50 years, whit mild to moderately
severe vertex MPHL according to a
modified Norwood/Hamilton
classification scale (II vertex, III
vertex, IV or V), were enrolled.
Women aged 18 to 50 years, with mild
to moderately severe vertex FPHL
according to a Ludwig classification
scale (I, II or III), were enrolled.
Principal exclusion criteria
included significant abnormalities
on screening physical examination or
laboratory evaluation, surgical
correction of scalp hair loss,
topical Minoxidil use within
one-year, use of drugs with
androgenetic or antiandrogenetic
properties, use of finasteride or
other 5αR inhibitors, or alopecia
due to other causes. Men and women
were instructed not to alter their
hairstyle or dye their hair during
the studies.
Study protocols
One initial, 6-months randomized, double-blind, placebo controlled studies were initiated, and both were continued as 1 consecutive, 6-months, double-bind, placebo-controlled extension studies. The objectives of the controlled extension studies were to determine the effect of long-term use of LDS 100, the effect of delaying treatment by one year, and the progression of MPHL in men and FPHL in women not receiving active treatment.
6-months initial studies
Following a screening procedure,
study subjects entered a 2 week,
single-blind, placebo run-in period.
All men and women received study
shampoo (Claim 3S®) for
standardization of shampoo used and
for prophylaxis of seborrheic
dermatitis, which might affect scalp
hair growth. Subjects (240 men and
80 women) were than randomized to
LDS 100 (2 times for week, 20
minutes, 140 Hz) or placebo LDS 100
(no infrared light, 2 times for
week, 20 minutes, 140 Hz) (1:1) for
six months (Figs. 1 and 2).
Men and women visited the clinic
every 3 months, where they completed
a hair growth questionnaire and
investigators completed assessments
of scalp hair growth.
Every 6 months, photographs of scalp
hair were taken for hair counts and
for the expert panel assessments of
hair growth. Reports of adverse
events were collected throughout the
studies.
6-months extension studies
Men and women completing the initial
6-months, placebo controlled studies
were eligible to enrol in one
consecutive, 6-months,
placebo-controlled extension studies.
In these extension studies, men (N =
183) and women (N = 55) were
randomly assigned (as determined at
initial randomization) to treatment
with either LDS 100 (2 times for
week, 20 minutes, 140 Hz) or placebo
LDS 100 (no infrared light, 2 times
for week, 20 minutes, 140 Hz) (9:1),
such that subjects were randomized
to one of four groups that allocated
treatment to them during both the
initial 6-months studies and the
6-months extension studies:
LDS 100
 LDS
100, LDS 100
LDS
100, LDS 100
 Placebo,
Placebo,
Placebo  LDS 100, or Placebo
LDS 100, or Placebo
 Placebo.
Placebo.
The procedures for the 6-months extension studies were similar to those for the initial 6-months studies.

Efficacy measurements
Four predefined efficacy endpoints
provided a comprehensive assessment
of changes in scalp hair from
baseline:
(1) hair counts, obtained from color
macrophotographs of a 1-inch
diameter circular area (5.1 cm2) of
clipped hair (length 1 mm), centered
at the anterior leading edge of the
vertex thinning area;
(2) patient self-assessment of scalp
hair, using a validated,
self-administered hair growth
questionnarie;
(3) investigator assessment of scalp
hair growth, using a standardized
7-point rating scale;
(4) independent assessment of
standardized clinical global
photographs of the vertex scalp by a
panel of dermatologists who were
blinded to treatment and experienced
in photagraphic assessment of hair
growth, using the standardized
7-point rating scale.
Safety
measurements
Safety measurements included
clinical and laboratory evaluations,
and adverse experience reports.
A data
analysis, plan pre-specified all
primary and secondary hypotheses,
including combining data from the
initial 6-months studies to improve
precision of the estimates of the
treatment effect, as well as from
each of the 6-months controlled
extensions due to the small size of
the placebo groups in those studies.
Hair counts were assessed by the
difference between the count at each
time point versus the baseline count,
and mean hair count values for each
treatment group were determined
using SASTM Least Squares Means.
Each of the seven questions in the
patient self-assessment of hair
growth was assessed separately, and
the responses to each question at
each time point were taken as
assessments of changes from baseline.
The investigator assessment of hair
growth and the expert panel
assessments of global photographs
were assessed by comparison of mean
rating scores for each tratment
group at each time point, based on
the 7-point rating scale (minimum
value = -3.0 [greatly decreased];
maximum value = 3.0 [greatly
increased].
Hypothesis testing for hair counts,
individual patient self-assessment
questions, and investigator and
global photographic assessments was
performed using analysis of variance.
The primary efficacy analysis
population for this report was the
intention to treat population, which
included all subjects with at least
one day randomozed therapy and with
both baseline and at least one
post-baseline efficacy assessment.
For all efficacy analyses, missing
data were estimated by carring data
forward from the previous visit.
However, no data were carried
forward from the baseline evaluation,
or between the initial 6-months
study and the 6-months extension
study.
A secondary population for analysis
of efficacy included only the data
from the cohort of subjects who
completed the 1-year study.
Safety analyses were based on all
subjects with at least one day of
randomized therapy. The safety
analyses focused on the biochemical
parameters, using analysis of
variance, and on adverse experience
reports.
Results
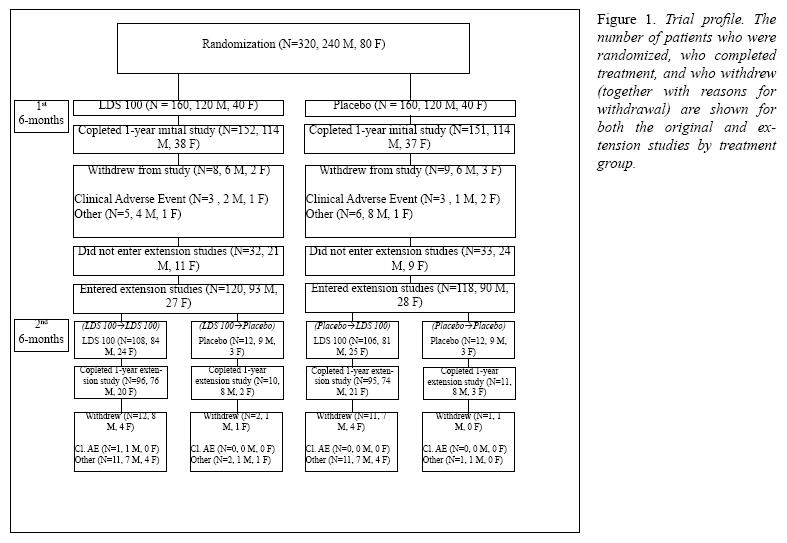
Patient accounting and baseline characteristicsPatient accounting is summarized in Figure 1. A summary of baseline characteristics for man and women who entered the extension study (2nd 6-months) is presented by treatment group in Table I. Demographics and baseline caharacteristics were comparable among the four treatment groups.
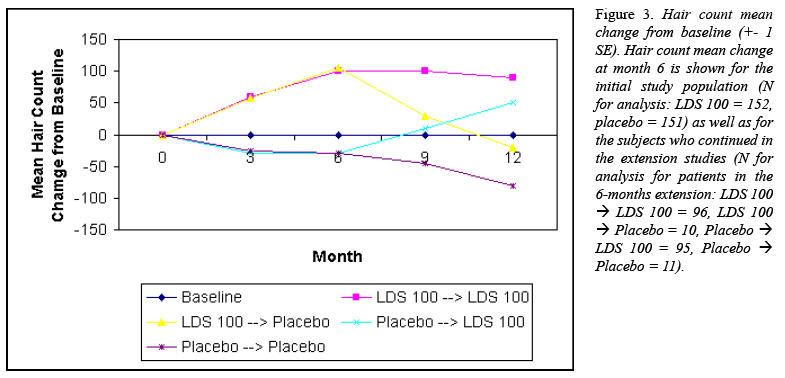
Hair countsIn the group
that received LDS 100 for all 12
months (LDS 100
 LDS
100), there were significant
increases in hair counts over 1 year
(p < 0.001 versus baseline for all
time points), which reached a
maximal increase at month 6,
declined somewhat thereafter but
remained above baseline throughout,
with a mean increase of hairs at
month 12 (Fig. 3).
LDS
100), there were significant
increases in hair counts over 1 year
(p < 0.001 versus baseline for all
time points), which reached a
maximal increase at month 6,
declined somewhat thereafter but
remained above baseline throughout,
with a mean increase of hairs at
month 12 (Fig. 3).
In contrast,
in the group that received placebo
for all 12 months (Placebo
 Placebo), there was a progressive
decline in hair counts over 1 year,
culminating in a mean decrease from
baseline of hairs at month 12 (Fig.
3).
Placebo), there was a progressive
decline in hair counts over 1 year,
culminating in a mean decrease from
baseline of hairs at month 12 (Fig.
3).
For the group
crossed over from Placebo to LDS 100
after 6 months (Placebo
 LDS
100) there was a decrease in hair
count during the months of placebo
treatment. This initial loss of hair
on placebo was followed by
significant increases in hair count
during treatment with LDS 100
through month 12 (Fig. 3). Increases
in hair count during LDS 100
treatment in this group were
generally sustained over time,
although the increases compared to
baseline were consistently less than
those observed in the LDS 100
LDS
100) there was a decrease in hair
count during the months of placebo
treatment. This initial loss of hair
on placebo was followed by
significant increases in hair count
during treatment with LDS 100
through month 12 (Fig. 3). Increases
in hair count during LDS 100
treatment in this group were
generally sustained over time,
although the increases compared to
baseline were consistently less than
those observed in the LDS 100
 LDS 100
group at comparable time points,
with the difference being similar in
magnitude to the amount of hair loss
sustained during the year of placebo
treatment.
LDS 100
group at comparable time points,
with the difference being similar in
magnitude to the amount of hair loss
sustained during the year of placebo
treatment.
For the group
that received LDS 100 for 1st 6
months, was crossed over to placebo
for 2nd 6 months (LDS 100
 Placebo), the beneficial effect on
hair count seen during the first
6-months of LDS 100 treatment was
reversed after 6 months of placebo
treatment (Fig. 3).
Placebo), the beneficial effect on
hair count seen during the first
6-months of LDS 100 treatment was
reversed after 6 months of placebo
treatment (Fig. 3).
For each of
the seven questions in the patient
self-assessment questionnaire,
treatment with LDS 100 (LDS 100
 LDS
100) was superior to treatment with
placebo (Placebo
LDS
100) was superior to treatment with
placebo (Placebo
 Placebo) at each time point (p <
0.001 for all between-group
comparisons).
Placebo) at each time point (p <
0.001 for all between-group
comparisons).
The LDS 100
 LDS 100
group demonstrated significant (p <
0.001) improvement from baseline at
each time point for each question,
with the exception that there was no
significant difference from baseline
at the month 6 time point for
Question 5a (assessment of
satisfaction with appearance of the
frontal hairline), where as the
Placebo
LDS 100
group demonstrated significant (p <
0.001) improvement from baseline at
each time point for each question,
with the exception that there was no
significant difference from baseline
at the month 6 time point for
Question 5a (assessment of
satisfaction with appearance of the
frontal hairline), where as the
Placebo  Placebo group generally demonstrated
deterioration from baseline over
time.
Placebo group generally demonstrated
deterioration from baseline over
time.
For each of the seven questions, a greater proportion of LDS 100- versus placebo-trated subjects reported an improvement from baseline, with the difference between groups increasing over time (Table II).
In the Placebo
 LDS 100
group, there was generally sustained
improvement following 6 months of
placebo treatment for each question
during the period of LDS 100
treatment (p < 0.001), although, as
with hair counts, this improvement
was less than that seen in the LDS
100
LDS 100
group, there was generally sustained
improvement following 6 months of
placebo treatment for each question
during the period of LDS 100
treatment (p < 0.001), although, as
with hair counts, this improvement
was less than that seen in the LDS
100  LDS
100 group at comparable time points.
LDS
100 group at comparable time points.
For the LDS
100  Placebo group, partial to complete
reversibility of the beneficial
effect of LDS 100 was observed for
six of the seven questions after 6
months of placebo treatment (2nd
6-months).
Placebo group, partial to complete
reversibility of the beneficial
effect of LDS 100 was observed for
six of the seven questions after 6
months of placebo treatment (2nd
6-months).

Based on the
investigator assessment, treatment
with LDS 100 (LDS 100
 LDS
100) was superior to treatment with
placebo (Placebo
LDS
100) was superior to treatment with
placebo (Placebo
 Placebo) at each time point (p <
0.001, all comparisons).
Placebo) at each time point (p <
0.001, all comparisons).
The Placebo
 LDS 100
group showed improvement during the
period of LDS 100 therapy, although
as with hair counts and patient
self-assessment the magnitude of
this improvement was less than that
seen in the LDS 100
LDS 100
group showed improvement during the
period of LDS 100 therapy, although
as with hair counts and patient
self-assessment the magnitude of
this improvement was less than that
seen in the LDS 100
 LDS 100
group at comparable time points.
LDS 100
group at comparable time points.
For the LDS 100
 Placebo
group, there was initial improvement
during the first year of LDS 100
treatment, followed by a plateau
during the 6-months of placebo
treatment.
Placebo
group, there was initial improvement
during the first year of LDS 100
treatment, followed by a plateau
during the 6-months of placebo
treatment.

Based on the
global photographic assessment,
treatment with LDS 100 (LDS 100
 LDS
100) was superior to treatment with
placebo (Placebo
LDS
100) was superior to treatment with
placebo (Placebo
 Placebo) at each time point (p <
0.001, all comparisons).
Placebo) at each time point (p <
0.001, all comparisons).
At month 12, 48% of LDS 100-treated subjects were rated as slightly, moderately, or greatly improved compared to 6% of placebo-treated subjects.
Viewed in the
context of maintaining visible hair
from baseline, 90% of subjects
treated with LDS 100 demonstrated no
further visible hair loss by this
assessment, compared to 25% of
patients on placebo.
Conversely, 75% of subjects treated
with placebo demonstrated futher
visible hair loss by this assessment
at 1 year, compared to 10% of
subjects on LDS 100 (Fig. 5).
For the LDS
100  LDS
100 group, maximal improvement by
global photographic assessment was
observed at month 12.
LDS
100 group, maximal improvement by
global photographic assessment was
observed at month 12.
In contrast,
the Placebo
 Placebo
group demonstrated progressive
worsening by global photographic
assessment through month 12.
Placebo
group demonstrated progressive
worsening by global photographic
assessment through month 12.
The Placebo
 LDS 100
group also demonstrated sustained
improvement in mean score during the
period of LDS 100 treatment from
month 6 to month 12 (p < 0.001),
although, as with the three other
efficacy measures, the magnitude of
improvement was less than that seen
in the LDS 100
LDS 100
group also demonstrated sustained
improvement in mean score during the
period of LDS 100 treatment from
month 6 to month 12 (p < 0.001),
although, as with the three other
efficacy measures, the magnitude of
improvement was less than that seen
in the LDS 100
 LDS 100
group for comparable time points.
LDS 100
group for comparable time points.
For the
Placebo  LDS 100 group, the beneficial effect
of LDS 100 was reversed after 6
months of placebo treatment (p <
0.001).
LDS 100 group, the beneficial effect
of LDS 100 was reversed after 6
months of placebo treatment (p <
0.001).
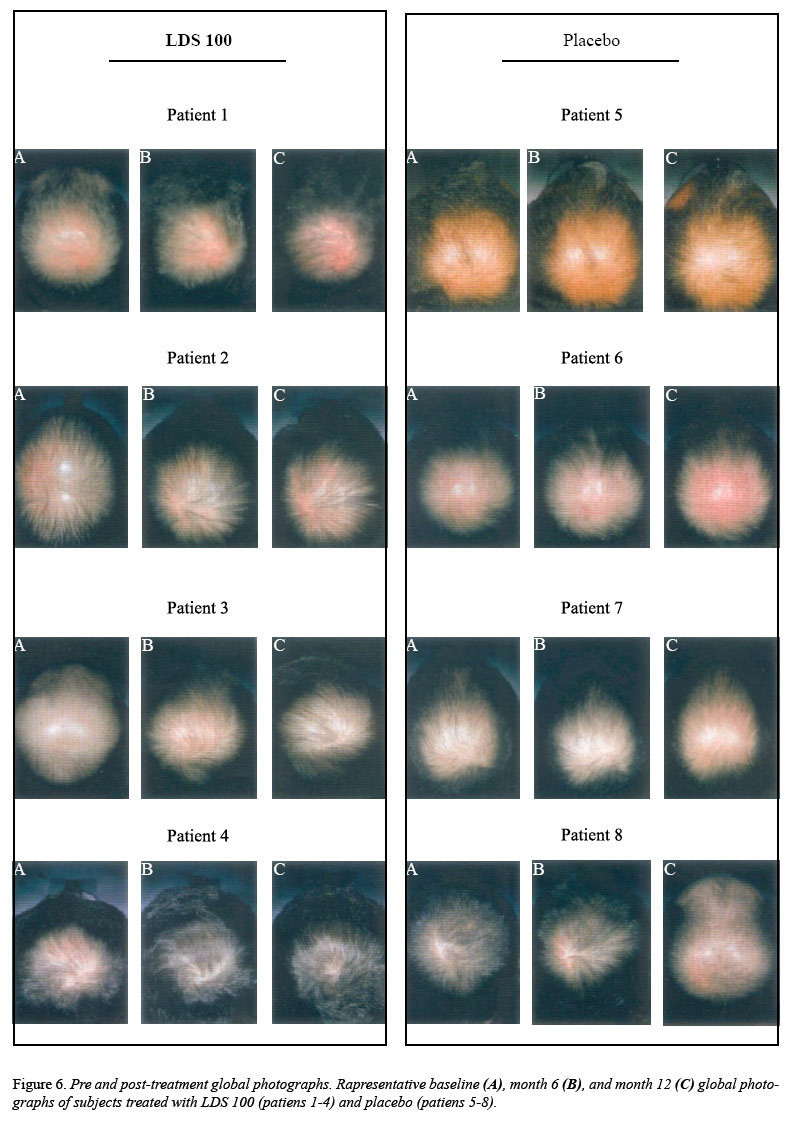
Global
photographs of representative
subjects from the Placebo
 Placebo
and LDS 100
Placebo
and LDS 100
 LDS 100
groups who were rated by the expert
panel as having decreased or
increased hair growth from baseline
are shown in Figure 6.
LDS 100
groups who were rated by the expert
panel as having decreased or
increased hair growth from baseline
are shown in Figure 6.

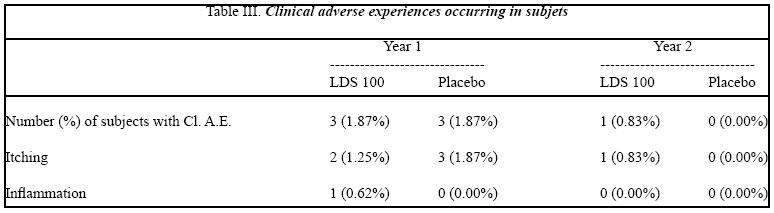
Clinical adverse experiences that were considered by the investigator to be possibly or definitely treatment-related and that occurred in at least 1% of subjects are summarized in Table III.
As reported previously, in the first 6-months a slightly higher proportion of LDS 100 than placebo subjects reported treatment-related adverse experiences related to itching and inflammation (Table III), discontinued the studies due these side effects. These side effects resolved after discontinuation and also resolved in most subjects who reported them but remained on therapy with LDS 100. The adverse experience profile for subjects continuing in the extension studies was similar to that of the initial studies (Table III).
Discussion
The data from this study and its long-term extension represent the longest reported controlled observations in men with MPHL and women with FPHL.
The combined analysis demonstrated that long-term tretament with LDS 100 led to significant and durable improvements, compared to both baseline and placebo, in scalp hair in men with MPHL and in women with FPHL. Hair counts increased over the first 6-months of treatment with LDS 100, with improvement above baseline maintained over 1 year. In contrast, the placebo group progressively lost hair over 1 year, confirming the natural progression of hair loss in this disorder due to the conyinued miniaturization of scalp hair. Thus, the treatment effect of LDS 100 on hair count relative to placebo increased progressively over time, leading to a net improvement for LDS 100-treated subjects hairs compared to placebo at one year. Most (65%) LDS 100-treated subjects had increases in hair counts at one year, compared to none of the placebo-treated subjects, but even for those LDS 100-treated subjects with less hair by hair count at one year, the magnitude of loss was less than that observed in the placebo group. these data support that the progression of hair loss observed in placebo-treated subjects was significantly reduced by treatment with LDS 100.
Based on the predefined endpoints utilizing photographic methods (hair counts, and global photographic assessment), peak efficacy was observed at 6 months to 12 months of treatment with LDS 100. This observation of an apparent peaking effect is likely due, in part, to the previously-reported beneficial effects of LDS 100 on the hair growth cycle based on a phototrichogram study. In that study, initiation of LDS 100 treatment was shown to increase the number of anagen-phase hairs and to increase the anagen to telogen ratio, consistent with normalization of the growth cycles of previously miniaturized hairs.
Consistent with these results, LDS 100 treatment was also shown to increase the growth rate and/or thikness of hairs, based on analysis of serial hair weight measurements. Because these beneficial changes in the hair growth cycle are dependent on when therapy with LDS 100 is initiated and occur rapidly, the affected hairs are driven to cycle in a synchronous manner. If these hairs have somewhat similar anagen phase durations, they would enter telogen phase as the anagen (and catagen) phase ended, followed by subsequent shedding, in a partially synchronized fashion. This would be expected to produce a gradual decline from peak hair count after a period of time equal to the average anagen phase duration. Eventually, as subsequent growth cycles recurred, these hairs would be expected to become increasingly independent, thereby losing their synchronous character as their growth cycles further normalized over time, leading to a sustained increase in hair count at a plateau above baseline, as suggested by the 1 year data presented here.
Patient self-assessment of hair growth provides a mechanism for each subject to judge the benefits of treatment under controlled and blinded conditions. This questionnaire asks specific questions about the patient’s hair growth or loss and his degree of satisfaction with the appearance of his hair compared to study start. While a placebo effect was observed with this instrument, as is typical of patient questionnaire data, results consistently demonstrated that subjects treated with LDS 100 had a more positive self-assessment of their hair growth and satisfaction with their appearance than subjects treated with placebo, with the majority of LDS 100-treated subjects reporting satisfaction with the overall appearance of their scalp hair at 1 year. Consistent with the findings of another study in which LDS 100 was evaluated in subjects with predominantly frontal MPHL, patients’ satisfaction with the appearance of their frontal hairline was improved by treatment with LDS 100 in the present study.
The investigators’ assessment are based on observations of subjects seen in the clinic and provide a clinically relevant assessment of the patient’s hair growth or loss since study start. These assessments demonstrated a sustained benefit of LDS 100 treatment over 1 year.
As with the
patient self-assessment, the
investigator assessment had a
greater placebo effect than the more
objective
endpoints of hair count and global
photographic assessment. Such an
effect is not inusual in
double-blind, placebo-control led
studies, and is often due to general
expectation bias on the part of the
patient’s treating physician.
Despite this
apparent placebo effect, the
beneficial effects of LDS 100 were
demonstrated by the clinical
assessment made by the investigators
in these studies. In contrast to the
investigator assessment, the blinded
comparison of paired pre- and
post-treatment global photographs by
the expert panel, which also
assessed change from baseline,
demonstrated minimal, if any,
placebo effect.
Based on this assessment, LDS 100
treatment led to maintenance of
improvement above baseline in scalp
hair growth and scalp coverage over
one year, while placebo subjects
progressively worsened.
Treatment with LDS 100 for one year led to sustained protection against further visible hair loss in nearly all (90%) subjects, while further visible hair loss as evident in most (75%) subjects treated with placebo over the same time period.
While the number of patients remaining in the study declined over time and the size of the placebo group was limited in the extension study, the results of analyses that included either all available patients at each time point or only the cohort of patients with data at month 12 were consistent and supported a sustained benefit in hair growth for subjects receiving LDS 100 compared with placebo.
Additionally, examination of data from placebo-treated subjects in all cohorts demonstrated the continued loss of scalp hair that occurs in untreated subjects with MPHL and FPHL. Thus, regardless of the cohort examined, the long-term data from these studies consistently demonstrated a beneficial effect of LDS 100 compared with placebo for men with MPHL and women with FPHL.
Moreover, this beneficial effect increased over time due to the progressive increase in the net treatment effect of LDS 100 compared with placebo.
The safety data from the one year of controlled observations in the current study provide reassurance that long-term use of LDS 100 in men with MPHL and women with FPHL is not associated with an increase in the incidence of adverse experiences or any new safety concerns.
A few subjects in the current studies experienced reversible itching and inflammation. No other significant adverse effects of LDS 100 were observed in the patient population evaluated in the current studies.
This excellent
safety profile of long-term use of
LDS 100 is consistent with the
experience with the infrared light
at five times the dose used in the
present study that has been
well-documented in large clinical
trials and post-marketing
surveillance in men and women.
In summary, treatment with LDS 100
over one year increased scalp hair
as determined by scalp hair counts,
patient self-assessment,
investigator assessment, and global
photographic assessment, when
compared with placebo. In contrast,
data from the placebo group
confirmed that without treatment
progressive reductions in hair count
and continued loss of visible hair
occurs.
Long-term
treatment with LDS 100 was generally
well tolerated. The results of these
studies demonstrate that chronic
therapy with LDS 100 leads to
durable improvements in hair growth
in men with MPHL and women with FPHL
and slows the further progression of
hair loss that occurs without
treatment.
References
1. Arndt
K.A., Noe I.M. et al.: Laser Therapy.
Basic concepts and nomenclature. J.
am. Acad. Dermatol. Dec. 1981, 5
2. Dwyer R.M., Bass M.: Laser
Applications. Vol. III Monte Ross
Ed. Academic press, N.Y., 1977.
3. Fine S., Klein E.: Interaction of
laser radiation with biological
system. Proceeding of the first
Conference on Biologic effects of
laser radiations. Fed. Proc., 24,
35, 1965.
4. Goldman L.: Effects of new laser
systems on the skin. Arch. Dermat..,
108, 385, 1973.
5. Goldman L.: Applications of the
Laser. Cleveland. Chemical Rubber
Co., 1973.
6. IIda H., Takahara H., Kahehi H.,
Tateno Y., Mammoto M.: Fundamental
studies on laser radiation therapy.
Nippon Acta radiol., 1969, Jul., 29,
411-5.
7. Mester E., Ludany G., Sellyei M.,
Szende B., Tota J.: The stimulating
effect of power laser rays on
biological systems. Laser Rev., 1,
3, 1968.
8. Mester E. et al.: Experimental
and clinical observations wih Laser.
Panmin. Med., 13, 538, 1971.
9. Olsen EA: Androgenetic alopecia.
In: Olsen EA, ed. Disorders of hair
growth: diagnosis and treatment. New
York: McGraw-Hill, Inc., 1994:
257-83.
10. Price VH: Testosterone
metabolism in the skin. Arch.
Dermatol. 1975; 111: 1496-502.
11. Wolbarsht M.L.: Laser
applications in medicine and biology.
Vol. 1-2 Plenum Press. N.Y.,
1971/74.
Table I. Baseline characteristics of
subjects entering the extension
study